Viscometry
Macromolecules increase the viscosity of a solution already at very low concentrations. The increase of the viscosity is not only dependent on the molecular weight but also dependent on the shape of the macromolecules. The shape and conformation of a random coil polymer depends on the solvent as well as on the temperature. It can be distinguished between the dynamic (η), kinematic (ν) and intrinsic viscosity ([η]):
η = ρ · ν
with ρ being the density of the solution. In practice, direct measurement of the dynamic viscosity is difficult, so that it is calculated from the easier obtainable kinematic viscosity and density.
The intrinsic viscosity [η] describes the contribution of a solute (polymer) to the overall viscosity of the solution. It can be obtained by determining the viscosity for solutions with different polymer concentration, linearization via the Huggins or Kraemer equation and subsequent extrapolation to concentration c = 0. The Mark-Houwink-equation relates the intrinsic viscosity to the molecular mass:
[η] = K·Mα
The log-log plot of [η] versus the molecular weight leads to a straight line with the slope α and the intercept log K. For many polymer/solvent systems the α- and K-values are listed in the literature (Polymer Handbook).
Determination of:
- dynamic (η) and kinematic (ν) viscosity of solutions and solvents
- intrinsic viscosity [η] and Mark-Houwink coefficients
Equipment:
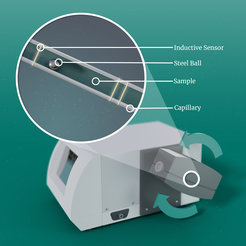
For viscosity measurements a Rolling-Ball viscometer and an Ubbelohde viscometer are available.
Literature:
| Contact: | Sandra Seywald | Christine Rosenauer | Ute Heinz |
| Phone: | 06131-379-227 | 06131-379-225 | 06131-379-227 |
