Isothermal Titration Calorimetry (ITC)
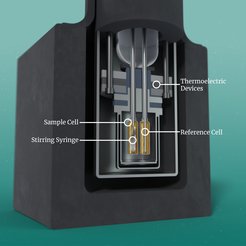
© MPIP/Schuhmacher
Isothermal titration calorimetry (ITC) is a method used to determine chemical and thermodynamic parameters of an interaction process between two components. Usually a measurement consists of an incremental titration, where a titrant is added stepwise to a sample of interest at certain time intervals. During this time the heat of the reaction being released or absorbed is being monitored via a power compensation measurement and then used to calculate binding affinities (Ka), reaction enthalpies (ΔH) and stoichiometries (N). This technique allows the examination of systems inside solution and therefore does not disturb any interactions through preparation methods.
Determination of:
- Association constant Ka
- Reaction enthalpy ΔH
- Reaction stoichiometry N
From those parameters the calculation of the Gibbs free energy ΔG and reaction entropy ΔS is possible. Thus, a complete thermodynamic characterization of a given binding process is possible. ITC is a nonstandard analysis technique, because each sample requires a method development.
Equipment / Sample requirements:
NanoITC Low Volume (TA Instruments)
- Only liquids (aqueous solution and organic solvents possible)
- No aggregation during the reaction
Literature:
Isothermal titration calorimetry as a complementary method for investigating nanoparticle–protein interactions
Nanoscale 11 (41), pp. 19265 - 19273 (2019)
Applications of isothermal titration calorimetry in pure and applied research—survey of the literature from 2010
Journal of Molecular Recognition, 2012, 25, 32-52
Biocalorimetry 2 – Applications of Calorimetry in the biological sciences
John Wiley & sons, Chichester, England, 2004, ISBN 0-470-84968-1
Isothermal titration calorimetry
Protein-Ligand interactions - Methods in Molecular Biology, 2005, 305, 1-15
| Contact: | Laila Chargui | Dr. Svenja Morsbach | |
| Phone: | 06131-379-532 | 06131-379-790 |
